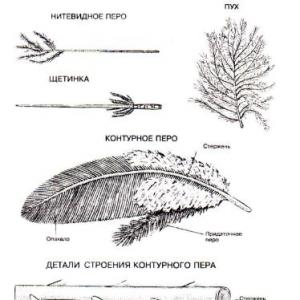
Production of sulfur dioxide from hydrogen sulfide. Chemistry tutor manual. Basic chemical properties of sulfur dioxide
Gas, colorless, with the smell of rotten eggs, poisonous, soluble in water (in 1 V H 2 O dissolves 3 V H 2 S at no.); t °pl. = -86°C ; t °b. = -60°C.
Effect of hydrogen sulfide on the body:
Hydrogen sulfide not only smells bad, it is also extremely toxic. When this gas is inhaled in large quantities, paralysis of the respiratory nerves quickly occurs, and then the person ceases to smell - this is the mortal danger of hydrogen sulfide.
There are many cases of poisoning with harmful gases when the victims were workers repairing pipelines. This gas is heavier, so it accumulates in holes and wells, from where it is not so easy to get out quickly.
Receipt
1) H 2 + S → H 2 S (at t)
2) FeS + 2 HCl → FeCl 2 + H 2 S
Chemical properties
1) Solution H 2 S in water it is a weak dibasic acid.
Dissociation occurs in two stages:
H 2 S → H + + HS - (first stage, hydrosulfide ion is formed)
HS - → 2 H + + S 2- (second stage)
Hydrogen sulfide acid forms two series of salts - medium (sulfides) and acidic (hydrosulfides):
Na 2 S– sodium sulfide;
CaS– calcium sulfide;
NaHS– sodium hydrosulfide;
Ca( H.S.) 2 – calcium hydrosulfide.
2) Interacts with bases:
H 2 S + 2 NaOH (excess) → Na 2 S + 2 H 2 O
H 2 S (excess) + NaOH → Na H S + H 2 O
3) H 2 S exhibits very strong restorative properties:
H 2 S -2 + Br 2 → S 0 + 2HBr
H 2 S -2 + 2FeCl 3 → 2FeCl 2 + S 0 + 2HCl
H 2 S -2 + 4Cl 2 + 4H 2 O →H 2 S +6 O 4 + 8HCl
3H 2 S -2 + 8HNO 3 (conc) → 3H 2 S +6 O 4 + 8NO + 4H 2 O
H 2 S -2 + H 2 S +6 O 4 (conc) →S 0 + S +4 O 2 + 2H 2 O
(when heated, the reaction proceeds differently:
H 2 S -2 + 3H 2 S +6 O 4 (conc) → 4S +4 O 2 + 4H 2 O
4) Hydrogen sulfide is oxidized:
in case of shortage O 2
2 H 2 S -2 + O 2 → 2 S 0 + 2 H 2 O
with excess O 2
2H 2 S -2 + 3O 2 → 2S +4 O 2 + 2H 2 O
5) Silver turns black when in contact with hydrogen sulfide:
4 Ag + 2 H 2 S + O 2 → 2 Ag 2 S ↓ + 2 H 2 O
Darkened objects can be restored to shine. To do this, they are boiled in an enamel bowl with a solution of soda and aluminum foil. Aluminum reduces silver to metal, and the soda solution retains sulfur ions.
6) Qualitative reaction for hydrogen sulfide and soluble sulfides - formation of a dark brown (almost black) precipitate PbS:
H 2 S + Pb(NO 3) 2 → PbS↓ + 2HNO 3
Na 2 S + Pb(NO 3) 2 → PbS↓ + 2NaNO 3
Pb 2+ + S 2- → PbS ↓
Atmospheric pollution causes blackening of the surface of paintings painted with oil paints that contain lead white. One of the main reasons for the darkening of artistic paintings by old masters was the use of lead white, which over several centuries, interacting with traces of hydrogen sulfide in the air (formed in small quantities during the rotting of proteins; in the atmosphere of industrial regions, etc.) turns into PbS. Lead white is a pigment that is lead carbonate ( II). It reacts with hydrogen sulfide contained in the polluted atmosphere, forming lead sulfide ( II), black connection:
PbCO 3 + H 2 S = PbS↓ + CO 2 + H 2 O
When processing lead sulfide ( II) with hydrogen peroxide the reaction occurs:
PbS + 4 H 2 O 2 = PbSO 4 + 4 H 2 O,
this produces lead sulfate ( II), the connection is white.
This is how blackened oil paintings are restored.
7) Restoration:
PbS + 4 H 2 O 2 → PbSO 4 (white) + 4 H 2 O
Sulfides
Preparation of sulfides
1) Many sulfides are prepared by heating the metal with sulfur:
Hg + S → HgS
2) Soluble sulfides are obtained by the action of hydrogen sulfide on alkalis:
H 2 S + 2 KOH → K 2 S + 2 H 2 O
3) Insoluble sulfides are obtained by exchange reactions:
CdCl 2 + Na 2 S → 2NaCl + CdS↓
Pb(NO 3) 2 + Na 2 S → 2NaNO 3 + PbS↓
ZnSO 4 + Na 2 S → Na 2 SO 4 + ZnS ↓
MnSO 4 + Na 2 S → Na 2 SO 4 + MnS ↓
2SbCl 3 + 3Na 2 S → 6NaCl + Sb 2 S 3 ↓
SnCl 2 + Na 2 S → 2NaCl + SnS↓
Chemical properties of sulfides
1) Soluble sulfides are highly hydrolyzed, as a result of which their aqueous solutions have an alkaline reaction:
K 2 S + H 2 O → KHS + KOH
S 2- + H 2 O → HS - + OH -
2) Sulfides of metals located in the voltage series to the left of iron (inclusive) are soluble in strong acids:
ZnS + H 2 SO 4 → ZnSO 4 + H 2 S
3) Insoluble sulfides can be converted into a soluble state by the action of concentrated HNO 3 :
FeS 2 + 8HNO 3 → Fe(NO 3) 3 + 2H 2 SO 4 + 5NO + 2H 2 O
ASSIGNMENT TASKS
Task No. 1Write the reaction equations that can be used to carry out the following transformations:
Cu→ CuS→ H2S→ SO 2
Task No. 2
Write down equations for the redox reactions of complete and incomplete combustion of hydrogen sulfide. Arrange the coefficients using the electronic balance method, indicate the oxidizing agent and reducing agent for each reaction, as well as the processes of oxidation and reduction.
Task No. 3
Write down the equation for the chemical reaction of hydrogen sulfide with a solution of lead (II) nitrate in molecular, total and short ionic form. Note the signs of this reaction, is the reaction reversible?
Task No. 4
Task No. 5
Determine the volume of hydrogen sulfide (n.s.) formed during the interaction of hydrochloric acid with a 25% solution of iron (II) sulfide weighing 2 kg?
Sulfuric acid is one of the main large-scale products of the chemical industry. It is used in various sectors of the national economy, since it has a set of special properties that facilitate its technological use. Sulfuric acid does not smoke, is colorless, odorless, and is in a liquid state at ordinary temperatures. In concentrated form it does not corrode ferrous metals. At the same time, sulfuric acid is one of the strong mineral acids, forms numerous stable salts and is cheap. Anhydrous sulfuric acid (monohydrate) H2SO4 is a heavy oily liquid that mixes with water in all proportions, releasing a large amount of heat.
Process raw materials: sulfur pyrites, elemental sulfur, hydrogen sulfide, metal sulfides such as copper pyrite CuFeS 2 , copper luster CuS 2 , sulfates:gypsum CaSO 4 2H 2 O, anhydrite CaSO 4 , mirabilite Na 2 SO 4 10H 2 O etc.
The production of gas sulfur from hydrogen sulfide, extracted during the purification of combustible and process gases, is based on the process of incomplete oxidation on a solid catalyst. In this case the following reactions occur:
H 2 S + 1.5O 2 = SO 2 + H 2 O;
2H 2 S + SO 2 = 2H 2 O + 1.5S 2.
Significant quantities of sulfur can be obtained from by-products of the production of non-ferrous metals, such as copper:
2FeS 2 = 2FeS +S 2;
SO 2 + C = S + CO 2;
CS 2 + SO 2 = 1.5S 2 + CO 2;
2COS + SO 2 = 1.5S 2 + 2CO 2
Production of sulfur dioxide by burning sulfur, hydrogen sulfide and other types of raw materials
When 1 mole of sulfur is burned, 1 mole of oxygen is consumed. This produces 1 mole of sulfur dioxide:
S (gas) + O2 (gas) = S02 (gas)-j - 362.4 kJ (86.5 kcal).
Therefore, when sulfur burns in air containing 21% oxygen, it is possible (theoretically) to obtain 21% sulfur dioxide. The yield of sulfur dioxide here is higher than when burning pyrites and zinc blende. By burning sulfur to produce sulfuric acid, the most favorable ratio of SO2 and oxygen is obtained. If you burn sulfur with a small excess of air, you can get sulfur dioxide with increased content S02. However, in this case the temperature develops up to 1300°C, which leads to the destruction of the furnace lining; this limits the production of gas with a high concentration of S02 from sulfur.
Hydrogen sulfide burns to form S02 and H20:
2H2S + 302 = 2S02+2H20-f 1038.7 kJ (247.9 kcal).
The water vapor formed in this case enters the contact apparatus with the gas mixture, and from it for absorption.
In terms of technological design, the production of sulfuric acid from iron pyrites is the most complex process and consists of several sequential stages.
The schematic diagram of this production is shown in the figure.
1 – production of roasting gas: 1 – roasting of pyrites, 2 – cooling of gas in a recovery boiler, 3 – general gas purification, 4 – special gas purification; 11 – contacting: 5 – heating of gas in the heat exchanger, 6 – contacting; 111 – absorption: 7 – absorption of sulfur oxide (6) and formation of sulfuric acid.

Sulfur dioxide S02 is a colorless gas, 2.3 times heavier than air, with a pungent odor. When dissolved in water, weak and unstable sulfurous acid SO2 + H2O = H2SO3 is formed.
2. Coal. Getting coke.
Coking of hard coals
A significant part of coals is subjected to high-temperature (pyrogenetic) chemical processing. The purpose of such processing is the production of valuable secondary products, which are further used as fuel and intermediate products for organic synthesis. According to the purpose and conditions, the processes of pyrogenetic processing of coal are divided into three types: pyrolysis, gasification, hydrogenation.
Pyrolysis or dry distillation is the process of heating solid fuel without air access in order to obtain gaseous, liquid and solid products for various purposes. Exists high temperature pyrolysis (coking) And low temperature pyrolysis (semi-coking).
Semi-coking carried out at 500–580 o C in order to obtain artificial liquid and gaseous fuel. The products of semi-coking are raw materials for organic synthesis, tar (a source of motor fuels), solvents, monomers and semi-coke, used as local fuel and an additive to the coking charge.
Processes hydrogenation And gasification are used to produce liquid products from coal used as motor fuel and combustible gases.
Coking of hard coal carried out at a temperature of 900 - 1200 o C in order to obtain coke, flammable gases and raw materials for the chemical industry.
Enterprises that coke coal are called coke plants. There are separate coke-chemical plants with a full cycle of coke-chemical production, located separately from metallurgical enterprises, and coke-chemical workshops as part of metallurgical plants.
The structural diagram of coke production is shown in the figure.
Coal
Coal preparation
Coal charge
Coke
Coking
HydrogenOCG
PKG Coke to warehouse
Cooling and separation
SB KUS
Overclocking
Overclocking
Individual arenas of the KUS Faction
Neutralization
for processing
Sulfuric acid
Ammonium sulfate
Fig. Block diagram of coke production
The diagram shows: OKG – reverse coke oven gas; PKG – direct coke oven gas; KUS – coal tar; SB – crude benzene.
According to its physicochemical nature, coking is a complex two-phase endothermic process in which thermophysical transformations of the coked raw material and secondary reactions occur with the participation of organic intermediates of the first stage of coking. Coking of coal is carried out in batch coke ovens, in which heat is transferred to the coked coal charge through the reactor wall.
3. Obtaining hydrochloric acid. Hydrochloric acid(hydrochloride, hydrochloride, hydrogen chloride) - HCl, a solution of hydrogen chloride in water; strong monoprotic acid. Colorless (technical hydrochloric acid is yellowish due to impurities of Fe, Cl 2, etc.), “smoking” in air, caustic liquid. The maximum concentration at 20 °C is 38% by weight, the density of such a solution is 1.19 g/cm³. Molar mass 36.46 g/mol. Salts of hydrochloric acid are called chlorides. Let's consider the main areas of acid use:
Metallurgy. Technical hydrochloric acid used for stripping metals during tinning and soldering. Also hydrochloric acid used in the production of manganese, iron and other substances.
Electrotype. In this direction technical hydrochloric acid acts as an active medium during etching and pickling.
Food industry. All kinds of acidity regulators, for example, E507, contain acid. And it’s hard to imagine soda (seltzer) water without such a substance as hydrochloric acid.
Medicine. In this area, of course, it is not used technical hydrochloric acid, and purified analogues, however, a similar phenomenon still occurs. In particular, we are talking about adding a substance to gastric juice in case of insufficient acidity.

In an adiabatic absorption column, hydrochloric acid of reduced concentration is obtained, but free of organic impurities. Acid with a higher concentration of HCI is produced in an isothermal absorption column at a reduced temperature. The degree of extraction of HCI from waste gases when dilute acids are used as absorbents is 90-95%. When pure water is used as an absorbent, the degree of extraction is almost complete.
4. Direct synthesis of concentrated nitric acid.
Direct synthesis of HNO 3 is based on the interaction of liquid nitrogen oxides with water and gaseous oxygen under pressure up to 5 MPa according to the equation
2N 2 O 4 + O 2 + 2H 2 O → 4HNO 3
100% nitrogen dioxide at atmospheric pressure and a temperature of 21.5 ° C completely transforms into a liquid state. During the oxidation of ammonia, the resulting NO is oxidized into NO 2, the content of which in the gas mixture is about 11%. It is not possible to convert nitrogen dioxide of such a concentration into a liquid state at atmospheric pressure, so increased pressure is used to liquefy nitrogen oxides.
Concentration of nitric acid using water-removing substances. It is impossible to obtain concentrated nitric acid by distilling dilute acid. When boiling and distilling dilute nitric acid, it can only be evaporated to a content of 68.4% HNO 3 (azeotropic mixture), after which the composition of the distilled mixture will not change.
In industry, the distillation of dilute aqueous solutions of nitric acid is carried out in the presence of water-removing substances (concentrated sulfuric acid, phosphoric acid, concentrated solutions of nitrates, etc.). The use of water-removing substances makes it possible to reduce the content of water vapor above the boiling mixture and increase the content of nitric acid vapor, upon condensation of which 98% HNO 3 is obtained.
Technological scheme for concentrating nitric acid using sulfuric acid:

Figure – Scheme for concentrating dilute nitric acid in the presence of sulfuric acid:
1, 4 – pressure tanks for nitric and sulfuric acid; 2 – control lights; 3 – evaporator of diluted nitric acid; 5 – box for regulating the supply of acid; 6 – concentration column; 7 – refrigerator condenser; 8 – cooler for acid circulating in the tower; 9 – fan: 10 – absorption tower; 11 – collection; 12 – pump; 13 – cooler for concentrated nitric acid, 14 – cooler for spent sulfuric acid
Dilute nitric acid from pressure tank 1 is supplied to column 6 through two flow meters 2 connected in parallel. One stream of acid passes into the evaporator 3 and is supplied as a mixture of liquid and steam to the 10th plate of the column 6, another stream without heating enters the overlying plate.
Sulfuric acid from the pressure tank 4 through the regulator 5 is supplied to the upper part of the column 6 above the input of the cold flow of nitric acid. IN bottom part Live steam is introduced into the column, and when heated, nitric acid begins to evaporate from the ternary mixture.
Nitric acid vapor at a temperature of 70–85 °C, rising upward, exits through a fitting in the column lid and enters refrigerator-condenser 7. These vapors contain impurities of nitrogen oxides and water.
In a refrigerator-condenser, nitric acid vapors at a temperature of about 30 ° C condense to form 98–99% HNO 3, while nitrogen oxides are partially absorbed by this acid. Concentrated nitric acid containing nitrogen oxides is directed to the two upper plates and passes them in series, and the oxides are blown out of the solution by nitric acid vapors entering condenser 7. Non-condensed nitric acid vapors and released nitrogen oxides are sent for absorption into tower 10, irrigated with water. The resulting 50% acid enters collection 11 and is again sent for concentration. After cooling, concentrated nitric acid is sent to the finished product warehouse.
Spent sulfuric acid containing 65–85% H 2 SO 4 is supplied for concentration. When concentrating nitric acid using 92–93% sulfuric acid, the consumption of the latter is significantly reduced when 59–60% HNO 3 is supplied for concentration instead of 48–50%. Therefore, in some cases it is advantageous to pre-concentrate 50% HNO 3 to 60% by simple evaporation.
A big disadvantage of concentrating nitric acid with sulfuric acid is the high content of H 2 SO 4 vapors and mist in the exhaust gases after electrostatic precipitators (0.3–0.8 g/m 3 of gas). Therefore, sulfuric acid is replaced, for example, with magnesium or zinc nitrate.
5. Obtaining ceramics.
Ceramics is a compositionally extensive group of dielectric materials, united by a common technological cycle. Currently, the word ceramics refers not only to clay-containing materials, but also to other inorganic materials with similar properties, the manufacture of products from which requires high-temperature firing. Source materials. Various natural and artificial materials are used to make ceramic products.
Artificial and natural materials - oxides, salts differ in the quantitative and qualitative content of impurities of foreign oxides and, in accordance with this, are conventionally designated by the letters: Ch (pure), analytical grade (pure for analysis), ChCh (chemically pure), etc. The original is also distinguished raw materials according to physical and chemical parameters (size and shape of particles, specific surface area, activity, etc.).
The starting raw material for the production of radio and piezoceramics is a large number of various salts and oxides: kaolins, clays, feldspars, silicon-containing materials, talcs - natural plastic materials; artificial non-plastic materials produced by industry - technical alumina and corundum, zirconium and titanium dioxides, beryllium oxide, barium and strontium carbonates.
Clays and kaolins consist predominantly of hydroaluminosilicates (Al 2 O 3 *2SiO 2 *H 2 O) and admixtures of iron salts, alkali and alkaline earth oxides and salts. Of the feldspars, the most suitable for the production of ceramics are potassium-sodium feldspars (K 2 O*Al 2 O 3 *6SiO 2 ; Na 2 O*Al 2 O 3 *6SiO 2 ). The basis of silicon-containing materials and quartz is silicon dioxide (SiO 2), which can contain various additives (iron oxides, clays, feldspars, etc.). The composition of talcs is varied: from 3MgO*4SiO 2 *H2O to 4MgO*5SiO 2 * H2O, impurities in them Fe 2 O 3, Al 2 O 3, CaO, Na 2 O, Cr 2 O, etc. The most undesirable impurities in all natural plastic materials are iron salts.
The named natural plastic materials are used to improve the plastic properties of press materials for molding products and as glass-forming additives in radioceramics. Talcs are the basis of such types of radioceramics as steatite and forsterite.
Technical alumina and corundum obtained by chemical processing of the natural raw material bauxite mineral and calcining it to 1100–1200 0 C. Zirconium dioxide (Zr 2 O 2), titanium (TiO 2), tin (SnO 2), beryllium oxides (B 2 O), strontium ( SrO), zinc (ZnO), lead (PbO), magnesium (MgO) are obtained by influencing the feedstock through a complex of chemical and thermal interactions.
Obtaining ceramics. The structure of ceramics is a complex system consisting of three main phases: crystalline, glassy and gaseous. The crystalline phase (main) represents chemical compounds or solid solutions; it determines the characteristic properties of the ceramic material; the glassy phase is found in the ceramic material in the form of layers between the crystalline component or separate microparticles and acts as a binder; the gas phase consists of gases contained in the pores of the ceramic. Pores worsen the properties of ceramics, especially at high humidity.
The properties of ceramics depend on the composition of the mixture (chemical and percentage ratio of substances) and processing mode.
Ceramics can be made by firing once or twice. This has its advantages and disadvantages.
In the production of ceramics, the following technological methods for manufacturing piezoceramics are common based on:
1) mechanical mixing of starting substances in the form of powders of metal oxides and salts corresponding to the chemical composition of the material being manufactured;
2) thermal decomposition of metal salts;
3) joint precipitation of carbonates of salts of the corresponding metals or their hydrates.
The starting materials for the manufacture of radio-piezoelectric ceramics and ferrites are metal oxides and salts. The main stages of the technological process are as follows.
The set of starting substances is determined by the specified magnetic and electrical properties of the products, geometric shape and dimensions.
Analysis of the original oxides and salts is carried out in order to determine their physical and chemical characteristics, the type and amount of impurities, the size and shape of particles, activity, i.e. the ability to react with other components of the mixture, etc.
Calculation of the mass and ratio of the starting components is carried out based on the chemical formula of the material. And then, in accordance with the calculation, the initial components are weighed.
Grinding or dissolving and mixing is performed to obtain a mixture that is homogeneous in chemical composition and particle size. These operations are performed either with liquid (water) or without water, i.e. Perform wet (slip) or dry grinding. Wet grinding is completed by drying.
The briquetting (granulation) operation is needed to obtain a more compact form of the resulting mixture (charge) and a more complete reaction during the next operation. Here briquettes, tablets or granules are obtained.
Preliminary firing of the charge is carried out for partial or complete diffusion processes between the oxides to transform them into ceramic material (ceramic synthesis) and reduce shrinkage during final firing.
Secondary grinding and mixing of briquettes, tablets or granules is carried out in order to obtain products with uniform properties, complete diffusion processes and provide the possibility of forming the product. The operation is performed in water or without water, and therefore after its completion, as in the first case, the resulting mixture is dried.
To improve the molding of powders, plasticizers (binders, lubricants) are introduced into them to improve the adhesion of individual particles. The introduction of plasticizers makes it possible to obtain various masses: for pressing - press powders, for casting - slips, and for forming from plastic masses - plastic masses.
The main methods of formation are pressing, molding from plastic masses, and slip casting.
The molded products are subjected to high-temperature sintering, during which a complex of certain magnetic, electrical, mechanical properties and physical and mechanical characteristics corresponding to the given material (radio-, piezoceramics, ferrite) is obtained.
6. Preparation of sodium hydroxide. Sodium hydroxide can be produced industrially by chemical and electrochemical methods.
Chemical properties
Physical properties
Under normal conditions, hydrogen sulfide is a colorless gas with a strong, characteristic odor of rotten eggs. T pl = -86 °C, T kip = -60 °C, poorly soluble in water, at 20 °C 2.58 ml of H 2 S dissolves in 100 g of water. Very toxic, if inhaled it causes paralysis, which can be fatal. In nature, it is released as part of volcanic gases and is formed during the decay of plant and animal organisms. It is highly soluble in water; when dissolved, it forms weak hydrosulfide acid.
- In an aqueous solution, hydrogen sulfide has the properties of a weak dibasic acid:
H 2 S = HS - + H + ;
HS - = S 2- + H + .
- Hydrogen sulfide burns in the air blue flame. With limited air access, free sulfur is formed:
2H 2 S + O 2 = 2H 2 O + 2S.
With excess air supply, combustion of hydrogen sulfide leads to the formation of sulfur oxide (IV):
2H 2 S + 3O 2 = 2H 2 O + 2SO 2.
- Hydrogen sulfide has reducing properties. Depending on conditions, hydrogen sulfide can be oxidized in aqueous solution to sulfur, sulfur dioxide and sulfuric acid.
For example, it decolorizes bromine water:
H 2 S + Br 2 = 2HBr + S.
interacts with chlorine water:
H 2 S + 4Cl 2 + 4H 2 O = H 2 SO 4 + 8HCl.
A stream of hydrogen sulfide can be ignited using lead dioxide, since the reaction is accompanied by a large release of heat:
3PbO 2 + 4H 2 S = 3PbS + SO 2 + 4H 2 O.
- Interaction of hydrogen sulfide with sulfur dioxide used to obtain sulfur from waste gases of metallurgical and sulfuric acid production:
SO 2 + 2H 2 S = 3S + 2H 2 O.
The formation of native sulfur during volcanic processes is associated with this process.
- When sulfur dioxide and hydrogen sulfide are simultaneously passed through an alkali solution, thiosulfate is formed:
4SO 2 + 2H 2 S + 6NaOH = 3Na 2 S 2 O 3 + 5H 2 O.
- Reaction of dilute hydrochloric acid with iron (II) sulfide
FeS + 2HCl = FeCl 2 + H 2 S
- Reaction of aluminum sulfide with cold water
Al 2 S 3 + 6H 2 O = 2Al(OH) 3 + 3H 2 S
- Direct synthesis from elements occurs when hydrogen is passed over molten sulfur:
H 2 + S = H 2 S.
- Heating a mixture of paraffin and sulfur.
1.9. Hydrogen sulfide acid and its salts
Hydrogen sulfide acid has all the properties of weak acids. It reacts with metals, metal oxides, bases.
As a dibasic acid, it forms two types of salts - sulfides and hydrosulfides . Hydrosulfides are highly soluble in water, sulfides of alkali and alkaline earth metals as well, and sulfides of heavy metals are practically insoluble.
Sulfides of alkali and alkaline earth metals are not colored, the rest have a characteristic color, for example, sulfides of copper (II), nickel and lead - black, cadmium, indium, tin - yellow, antimony - orange.
Ionic alkali metal sulfides M 2 S have a fluorite-type structure, where each sulfur atom is surrounded by a cube of 8 metal atoms and each metal atom is surrounded by a tetrahedron of 4 sulfur atoms. MS-type sulfides are characteristic of alkaline earth metals and have a sodium chloride-type structure, where each metal and sulfur atom is surrounded by an octahedron of atoms of a different type. As the covalent nature of the metal–sulfur bond increases, structures with lower coordination numbers are realized.
Sulfides of non-ferrous metals are found in nature as minerals and ores and serve as raw materials for the production of metals.
When 1 mole of sulfur is burned, 1 mole of oxygen is consumed. This produces 1 mole of sulfur dioxide:
S (gas) + Og (gas) = S02 (gas)-j - 362.4 kJ (86.5 kcal).
Therefore, when sulfur burns in air containing 21% oxygen, it is possible (theoretically) to obtain 21% sulfur dioxide. The yield of sulfur dioxide here is higher than when burning pyrites and zinc blende. By burning sulfur to produce sulfuric acid, the most favorable ratio of SO2 and oxygen is obtained. If you burn sulfur with a slight excess of air, you can get sulfur dioxide with a high SO2 content. However, at the same time, it develops at a pace
temperature up to 1300°C, which leads to destruction of the furnace lining; this limits the production of gas with a high concentration of S02 from sulfur.
Hydrogen sulfide burns to form S02 and H20:
2H2S + 302 = 2S02+2H20-f 1038.7 kJ (247.9 kcal).
The water vapor formed in this case enters the contact apparatus with the gas mixture, and from it for absorption.
The process of producing sulfuric acid by contact method, in which the oxidation of S02 to S03 and the absorption of S03 is carried out in the presence of water vapor, is called the wet catalysis method.
The maximum (theoretical) content of sulfur dioxide when burning hydrogen sulfide in air is about 13%.
When burning carbon pyrites, sulfur dioxide is produced, which contains carbon dioxide CO2. It is the result of the combustion of carbon, which is part of the carbon pyrite: C + 02 = C02.
The combustion of carbon consumes oxygen from the air, which leads to a decrease in the oxygen concentration in the combustion gas; As already indicated, oxygen in the roasting gas is necessary for the oxidation of S02 and S03. To reduce the amount of carbon, carbon pyrite is enriched. To do this, crushed pyrite is washed with water, onto the surface of which lighter coal floats. Enriched carbon pyrite contains 3-6% carbon. In fluidized bed kilns, combustion of carbon pyrite does not cause difficulties, so the requirements for the degree of its enrichment can be significantly reduced.
The use of phosphogypsum to produce sulfuric acid is of great economic importance, since the production of phosphoric acid and concentrated phosphorus and complex fertilizers consumes a large amount of sulfuric acid, which is excreted in the form of phosphogypsum. Gypsum can also be used to produce sulfuric acid. In this case, gypsum or phosphogypsum is first heated to release water of crystallization:
CaS04 2H20 -> CaS04-|- 2H20 (39)
The resulting anhydrite CaS04 decomposes upon further heating:
CaS04=Ca0-f S02+1/202 - 489.6 kJ (116.86 kcal).
As can be seen from equation (40), this reaction is endothermic, i.e., it requires heat. Complete decomposition of anhydrite is achieved only at 1400-1500° C. This requires a lot of fuel. If coal is added to anhydrite during calcination, the decomposition temperature is reduced to 800-900 ° C and the process proceeds according to the reaction
2CaS04 + C = 2CaO + 2S02 + C02 - 566.2 kJ (135.12 kcal).
If clay (SiO2, Al203) and Fe2O3 are added to a mixture of CaS04 and coal, a cinder is formed, and when crushed, cement is obtained. In other words, when decomposing gypsum or phosphogypsum, in addition to the sulfur dioxide used for sulfuric acid, cement can also be obtained.
Due to their low concentration of S02, flue and sinter gases are not used for the production of sulfuric acid. However, the problem of their disposal is becoming increasingly acute, so it is necessary to develop cost-effective methods for their enrichment in order to obtain more concentrated sulfur dioxide, which could be used to produce sulfuric acid.
General information. There are furnaces for firing pyrites various designs: mechanical shelf (multi-hearth), rotating cylindrical, pulverized kilns, fluidized bed kilns. Pyrite is fired in mechanical shelf kilns...
Amelin A. G., Yashke E. V. As already mentioned, the main part of sulfuric acid is consumed for the manufacture of fertilizers. Plant nutrition especially requires phosphorus and nitrogen. Natural phosphorus compounds (apatites and...
Physico-chemical basis of the process. The process of oxidation of sulfur dioxide to sulfur dioxide proceeds according to the reaction 2S02 + 02^S03 + A^, (45) Where AH is the thermal effect of the reaction. Percentage ratio of the amount of S02 oxidized to S03 to ...
DEFINITION
Hydrogen sulfide is a colorless gas with a characteristic odor of rotting protein.
It is slightly heavier than air, liquefies at a temperature of -60.3 o C and solidifies at -85.6 o C. In air, hydrogen sulfide burns with a bluish flame, forming sulfur dioxide and water:
2H 2 S + 3O 2 = 2H 2 O + 2SO 2.
If you introduce some cold object, such as a porcelain cup, into the hydrogen sulfide flame, the temperature of the flame drops significantly and the hydrogen sulfide oxidizes only to free sulfur, which settles on the cup in the form of a yellow coating:
2H 2 S + O 2 = 2H 2 O + 2S.
Hydrogen sulfide is highly flammable; its mixture with air explodes. Hydrogen sulfide is very poisonous. Prolonged inhalation of air containing this gas, even in small quantities, causes severe poisoning.
At 20 o C, one volume of water dissolves 2.5 volumes of hydrogen sulfide. A solution of hydrogen sulfide in water is called hydrogen sulfide water. When standing in the air, especially in the light, hydrogen sulfide water soon becomes cloudy from the sulfur released. This occurs as a result of the oxidation of hydrogen sulfide by atmospheric oxygen.
Production of hydrogen sulfide
At high temperature sulfur reacts with hydrogen to form hydrogen sulfide gas.
In practice, hydrogen sulfide is usually produced by the action of dilute acids on sulfur metals, for example iron sulfide:
FeS + 2HCl = FeCl 2 + H 2 S.
More pure hydrogen sulfide can be obtained by hydrolysis of CaS, BaS or A1 2 S 3. The purest gas is obtained by the direct reaction of hydrogen and sulfur at 600 °C.
Chemical properties of hydrogen sulfide
A solution of hydrogen sulfide in water has the properties of an acid. Hydrogen sulfide is a weak dibasic acid. It dissociates step by step and mainly according to the first step:
H 2 S↔H + + HS - (K 1 = 6 × 10 -8).
Second stage dissociation
HS - ↔H + + S 2- (K 2 = 10 -14)
occurs to a negligible extent.
Hydrogen sulfide is a strong reducing agent. When exposed to strong oxidizing agents, it is oxidized to sulfur dioxide or sulfuric acid; the depth of oxidation depends on the conditions: temperature, pH of the solution, concentration of the oxidizing agent. For example, the reaction with chlorine usually proceeds to form sulfuric acid:
H 2 S + 4Cl 2 + 4H 2 O = H 2 SO 4 + 8HCl.
Medium salts of hydrogen sulfide are called sulfides.
Application of hydrogen sulfide
The use of hydrogen sulfide is quite limited, which is primarily due to its high toxicity. It has found application in laboratory practice as a precipitant for heavy metals. Hydrogen sulfide serves as a raw material for the production of sulfuric acid, sulfur in elemental form and sulfides
Examples of problem solving
EXAMPLE 1
| Exercise | Determine how many times heavier than air is hydrogen sulfide H 2 S. |
| Solution | The ratio of the mass of a given gas to the mass of another gas taken in the same volume, at the same temperature and the same pressure is called the relative density of the first gas to the second. This value shows how many times the first gas is heavier or lighter than the second gas. The relative molecular weight of air is taken to be 29 (taking into account the content of nitrogen, oxygen and other gases in the air). It should be noted that the concept of “relative molecular mass of air” is used conditionally, since air is a mixture of gases. D air (H 2 S) = M r (H 2 S) / M r (air); D air (H 2 S) = 34 / 29 = 1.17. M r (H 2 S) = 2 × A r (H) + A r (S) = 2 × 1 + 32 = 2 + 32 = 34. |
| Answer | Hydrogen sulfide H 2 S is 1.17 times heavier than air. |
EXAMPLE 2
| Exercise | Find the hydrogen density of a mixture of gases in which the volume fraction of oxygen is 20%, hydrogen is 40%, and the rest is hydrogen sulfide H 2 S. |
| Solution | The volume fractions of gases will coincide with the molar ones, i.e. with fractions of quantities of substances, this is a consequence of Avogadro’s law. Let's find the conditional molecular weight of the mixture: M r conditional (mixture) = φ (O 2) × M r (O 2) + φ (H 2) × M r (H 2) + φ (H 2 S) × M r (H 2 S); |