Stones of meteorite origin. Types of meteorites. Types of meteorites by chemical composition and structure
The main feature of meteorites is the so-called melting crust. It has a thickness of no more than 1 mm and covers the meteorite on all sides in the form of a thin shell. The black bark on stony meteorites is especially noticeable.
The second sign of meteorites is the characteristic pits on their surface. Meteorites usually come in the form of debris. But sometimes there are meteorites with a remarkable cone shape. They resemble a projectile head. This cone-shaped shape is formed as a result of the “sharpening” action of air.
The largest single meteorite was found in Africa in 1920. This meteorite is iron and weighs about 60 tons. Usually meteorites weigh several kilograms. Meteorites weighing tens, and even more so hundreds of kilograms fall very rarely. The smallest meteorites weigh fractions of a gram. For example, at the site of the fall of the Sikhote-Alin meteorite, the smallest specimen was found in the form of a grain weighing only 0.18 G; The diameter of this meteorite is only 4 mm.
Stone meteorites fall most often: on average, out of 16 meteorites that fall, only one turns out to be iron.
WHAT ARE METEORITES MADE OF?
By studying the chemical composition of meteorites, scientists have determined that meteorites consist of the same chemical elements that are found on Earth. No new elements were found in them.

The eight chemical elements most commonly found in meteorites are iron, nickel, sulfur, magnesium, silicon, aluminum, calcium and oxygen. All other chemical elements of the periodic table are found in meteorites in negligible, microscopic quantities. By combining chemically with each other, these elements form various minerals. Most of these minerals are found in terrestrial rocks. And in very insignificant quantities minerals were found in meteorites that do not and cannot exist on Earth, since it has an atmosphere with a high oxygen content. When they combine with oxygen, these minerals form other substances.
Iron meteorites are composed almost entirely of iron combined with nickel, while stony meteorites are composed primarily of minerals called silicates. They consist of compounds of magnesium, aluminum, calcium, silicon and oxygen.
Particularly interesting internal structure iron meteorites. Their polished surfaces become shiny like a mirror. If you etch such a surface with a weak acid solution, an intricate pattern usually appears on it, consisting of individual stripes and narrow edges intertwining with each other. On the surfaces of some meteorites, parallel thin lines appear after etching. All this is the result of the internal crystalline structure of iron meteorites.
The structure of stone meteorites is no less interesting. If you look at a fracture in a stone meteorite, you can often see even with the naked eye small round balls scattered across the surface of the fracture. These balls sometimes reach the size of a pea. In addition to them, scattered tiny shiny particles are visible in the fracture white. These are inclusions of nickel iron. Among such particles there are golden sparkles - inclusions of a mineral consisting of iron combined with sulfur. There are meteorites that look like an iron sponge, in the voids of which grains of the yellowish-green color of the mineral olivine are contained.
ORIGIN OF METEORITES
Most scientists believe that meteorites are fragments of one or (more likely) several large celestial bodies, similar to asteroids that previously existed in the solar system.

Soviet scientists - Academician V. G. Fesenkov, S. V. Orlov and others - believe that asteroids and meteorites are closely related to each other. Asteroids are giant meteorites, and meteorites are very small, dwarf asteroids. Both are fragments of planets that billions of years ago moved around the Sun between the orbits of Mars and Jupiter. These planets apparently fell apart as a result of the collision. Countless fragments of various sizes were formed, down to the smallest grains. These fragments are now carried in interplanetary space and, colliding with the Earth, fall onto it in the form of meteorites.
HELP OF THE POPULATION IN COLLECTING METEORITES
Meteorites always fall unexpectedly, and it is impossible to predict when and where it will happen. Therefore, specialists cannot prepare in advance for observations of meteorite falls. Meanwhile, the study of the movements of meteoric bodies in earth's atmosphere has very great scientific significance.
In addition, by observing the fireball, you can approximately determine the place where the meteorite could have fallen and search for it there. Therefore, the public can greatly help scientists in their work if eyewitnesses of the meteorite fall describe in detail all the phenomena that they noticed during the movement of the fireball and the fall of the meteorite to the Earth.
When receiving a large number of such descriptions made by eyewitnesses in different populated areas, it is possible to quite accurately determine the path of a meteoroid in the earth's atmosphere, the height of the appearance and disappearance of the fireball, as well as the inclination and direction of its path. Reports of meteorites should be sent to the Committee on Meteorites of the USSR Academy of Sciences.
If a meteorite is found, under no circumstances should it be crushed. It is necessary to take all measures to protect it and transfer it to the Committee on Meteorites.
When describing the fireball phenomenon, it is necessary, if possible, to answer the following questions: 1) date and time of the fall; 2) observation location; 3) direction of movement of the car; 4) duration of the car’s flight in seconds; 5) the size of the fireball compared to the apparent size of the Moon or Sun; 6) car color; 7) whether the area was illuminated during the flight of the car; 8) whether fragmentation of the car was observed; 9) whether there was a trace left behind the car; what is its form and subsequent change, as well as the duration of visibility; 10) what sounds were observed during the flight of the car and after its disappearance.
The description must also indicate the last name, first name, patronymic and address of the observer.
If you find an error, please highlight a piece of text and click Ctrl+Enter.
Origin of meteorites
Currently, many museums around the world store at least 500 tons of meteorite matter. Calculations show that about 10 tons of matter fall to the Earth in the form of meteorites and meteor dust per day, which over a period of 2 billion years gives a layer 10 cm thick.
The source of almost all small meteoric particles is apparently comets. Large meteoroids are of asteroid origin.
Russian scientists - academician V.G. Fesenkov, S.V. Orlov and others believe that comets and meteorites are closely related. Asteroids are giant meteorites, and meteorites are very small, dwarf comets. Both are fragments of planets that billions of years ago moved around the Sun between the orbits of Mars and Jupiter. These planets apparently fell apart as a result of the collision. Countless fragments of various sizes were formed, down to the smallest grains. These fragments are now carried in interplanetary space and, colliding with the Earth, fall onto it in the form of meteorites.
Composition of meteorites and their substances
In some cases, a large meteoroid body, while moving through the atmosphere, does not have time to evaporate and reaches the surface of the Earth. This remnant of a meteoric body is called a meteorite. Over the course of a year, approximately 2,000 meteorites fall on Earth.
Depending on the chemical composition, meteorites are divided into stony chondrites (their relative abundance is 85.7%), stony achondrites (7.1%), iron (5.7%) and stony-iron meteorites (1.5%). Chondrules are small round particles. gray, often with a brown tint, abundantly interspersed in the stone mass.
Iron meteorites consist almost entirely of nickel iron. From calculations it follows that the observed structure of iron meteorites is formed if, in the temperature range from approximately 600 to 400 C, the substance cools at a rate of 1 ° - 10 ° C per million years.
Stony meteorites that do not contain chondrules are called achondrites. The analysis showed that chondrules contain almost all chemical elements. The eight chemical elements most commonly found in meteorites are iron, nickel, sulfur, magnesium, silicon, aluminum, calcium and oxygen. All other chemical elements of the periodic table are found in meteorites in negligible, microscopic quantities. By combining chemically with each other, these elements form various minerals. Most of these minerals are found in terrestrial rocks. And in very insignificant quantities minerals were found in meteorites that do not and cannot exist on Earth, since it has an atmosphere with a high oxygen content. When they combine with oxygen, these minerals form other substances. Iron meteorites are composed almost entirely of iron combined with nickel, while stony meteorites are composed primarily of minerals called silicates. They consist of compounds of magnesium, aluminum, calcium, silicon and oxygen.
The internal structure of iron meteorites is especially interesting. Their polished surfaces become shiny like a mirror. If you etch such a surface with a weak acid solution, an intricate pattern usually appears on it, consisting of individual stripes and narrow edges intertwining with each other. On the surfaces of some meteorites, parallel thin lines appear after etching. All this is the result of the internal crystalline structure of iron meteorites. The structure of stone meteorites is no less interesting. If you look at a fracture in a stone meteorite, you can often see even with the naked eye small round balls scattered across the surface of the fracture. These balls sometimes reach the size of a pea. In addition to them, scattered tiny shiny white particles are visible in the fracture. These are inclusions of nickel iron. Among such particles there are golden sparkles - inclusions of a mineral consisting of iron combined with sulfur. There are meteorites that look like an iron sponge, in the voids of which grains of the yellowish-green color of the mineral olivine are contained.
Meteorites are divided into three large classes: iron, stony and stony-iron.
Iron meteorites are composed primarily of nickel iron. A natural alloy of iron and nickel does not occur in terrestrial rocks, so the presence of nickel in pieces of iron indicates its cosmic (or industrial!) origin.
Nickel iron inclusions are found in most stony meteorites, which is why space rocks tend to be heavier than terrestrial rocks. Their main minerals are silicates (olivines and pyroxenes). A characteristic feature of the main type of stony meteorites - chondrites - is the presence of round formations inside them - chondrules. Chondrules consist of the same substance as the rest of the meteorite, but stand out on its section in the form of individual grains. Their origin is not yet entirely clear.
The third class - stony-iron meteorites - are pieces of nickel iron interspersed with grains of stony minerals.
In general, meteorites consist of the same elements as terrestrial rocks, but combinations of these elements, i.e. minerals may also be those that are not found on Earth. This is due to the peculiarities of the formation of bodies that gave birth to meteorites.
Among the falls, stony meteorites predominate. This means that there are more such pieces flying in space. As for the finds, iron meteorites predominate here: they are stronger, better preserved in terrestrial conditions, and stand out more sharply against the background of terrestrial rocks.
Meteorite is a solid extraterrestrial substance that was preserved during its passage through the atmosphere and reached the surface of the Earth. Meteorites are the most primitive substance of the SS, which has not experienced further fractionation since its formation. This is based on the fact that the relative distribution refractory el. in meteorites corresponds to solar distribution. Meteorites are divided into (based on metal phase content): Stone(aerolites): achondrites, chondrites, Iron-stone(siderolites), Iron(Siderites). Iron meteorites – consist of kamacite - native Fe of cosmic origin with an admixture of nickel from 6 to 9%. Stone-iron meteorites Low spread group. They have coarse-grained structures with equal weight fractions of silicate and Fe phases. (Silicate minerals - Ol, Px; Fe phase - kamacite with Widmanstätten growths). Stone meteorites – consist of Mg and Fe silicates with an admixture of metals. Divided into Chondrite, achondrite and carbonaceous.Chondrites: spheroidal segregations of a few mm or less in size, composed of silicates, less often silicate glass. Immersed in a Fe-rich matrix. The main mass of chondrites is a fine-grained mixture of Ol, Px-s (Ol-bronzite, Ol-hypersthene and Ol-pigeonite) with nickel Fe (Ni-4-7%), troilite (FeS) and plagioclase. Chondrites are crystalline. or glassy drops, cat. Image. by melting pre-existing silicate material that has been subjected to heat. Achondrites: They do not contain chondrules and have a lower content. nickel Fe and coarser structures. Their main minerals are Px and Pl, some types are enriched in Ol. In composition and structural features, achondrites are similar to terrestrial Gabbroids. The composition and structure indicate igneous origin. Sometimes bubble structures like lavas are observed. Carbonaceous chondrites (large amounts of carbonaceous matter) A characteristic feature of carbonaceous chondrites is presence of a volatile component, which indicates primitiveness (volatile elements were not removed) and did not undergo fractionation. Type C1 contains a large number chlorite(aqueous Mg, Fe aluminosilicates), as well as magnetite, water-soluble salt, nativeS, dolomite, olivine, graphite, organ. connections. Those. from the moment of their image, they are beings. at T, not > 300 0 C. In the composition chondritic meteorites lack of 1/3 chemical Email compared to composition carbonaceous chondrites, cat. are closest to the composition of protoplanetary matter. The most likely cause of the shortage of volatile electricity. - sequential condensation of electricity. and their compounds in the reverse order of their volatility.
5.Historical and modern models of accretion and differentiation of protoplanetary matter O.Yu. Schmidt in the 40s expressed the idea that the Earth and the Earth planets were formed not from hot clumps of solar gases, but through the accumulation of TV. bodies and particles - planetesimals that experienced melting later during accretion (heating due to collisions of large planetesimals, up to a few hundred km in diameter). Those. early core-mantle differentiation and degassing. Noun relates two points of view. the mechanism of accumulation and ideas about the shape of the layered structure of planets. Models homogeneous and heterogeneous accretion: HETEROGENEOUS ACCRETION 1. Short-term accretion. Early heterogeneous accretion models(Turekian, Vinogradov) assumed that the earth was accumulated from material as it condensed from a protoplanetary cloud. Early models include early >T accumulation of the Fe-Ni alloy, forming the protocore of the earth, followed by lower. T by accretion of its outer parts from silicates. It is now believed that continuous change occurs during the accretion process. in the accumulating material, the Fe/silicate ratio from the center to the periphery of the forming planet. During accumulation, gold heats up, => melting of Fe, which is separated from silicates and sinks into the core. After the planet cools, about 20% of its mass is added by material enriched in volatiles along the periphery. In the proto-earth there were no sharp boundaries between the core and the mantle, cat. established as a result of gravitational and chem. differentiation at the next stage of the planet's evolution. In early versions, differentiation occurred mainly during the formation of the Earth's Earth, and did not cover the entire Earth. HOMOGENEOUS ACCRETION 2. A longer accretion time is accepted - 10 8 years. During the accretion of the Earth and the planets of the Earth, the condensing bodies had wide variations in composition from carbonaceous chondrites enriched in volatiles to materials enriched in refractory components of the Allende type. Planets of forms. from this set of meteorite objects and their differences and similarities were determined by reference. proportions of ingredients of different composition. The same happened macroscopic homogeneity of protoplanets. The existence of a massive core suggests that the alloy initially brought by Fe-Ni meteorites, uniformly distributed throughout the entire planet, was released into the central part during its evolution. Homogeneous in composition the planet split into shells in the process of gravitational differentiation and chemical processes. Modern model of heterogeneous accretion, allowing us to explain the chemistry. the composition of the mantle is being developed by a group of German scientists (Wencke, Dreybus, Jagoutz). They found that the contents of moderately volatile (Na, K, Rb) and moderately siderophilic (Ni, Co) elements in the mantle, with different The distribution coefficients Me/silicate have the same abundance (normalized by C1) in the mantle, and the most strongly siderophile elements have excess concentrations. Those. the core was not in equilibrium with the mantle reservoir. They proposed heterogeneous accretion :1. Accretion begins with the accumulation of a highly reduced component A, devoid of volatile elements. and containing all other emails. in quantities corresponding to C1, and Fe and all siderophiles in a reduced state. As T increases, core formation begins simultaneously with accretion. 2. After accretion, more and more oxidized material, component B, begins to accumulate in 2/3 of the earth’s mass. Part of the Me component of component A is still preserved and contributes to the extraction of the most siderophilic elements. and transfer them to the nucleus. Source of moderately volatile, volatile and moderately siderophilic el. in the mantle component B, which explains their close relative prevalence. Thus, the Earth consists of 85% component A and 15% B. In general, the composition of the mantle is formed after the separation of the core by homogenization and mixing of the silicate part of component A and the substance of component B.
6. Isotopes of chemical elements. Isotopes - atoms of the same electron, but having a different number of neutrons N. They differ only in mass. Isotones - atoms of different elements, having different Z, but the same N. They are located in vertical rows. Isobars - atoms of different elements, cat. equal mass. numbers (A=A), but different Z and N. They are located in diagonal rows. Nuclear stability and isotope abundance; radionuclides The number of known nuclides is ~ 1700, of which ~ 260 are stable. On the nuclide diagram, stable isotopes (shaded squares) form a band surrounded by unstable nuclides. Only nuclides with a certain ratio of Z and N are stable. The ratio of N to Z increases from 1 to ~ 3 with increasing A. 1. Nuclides that have a cat are stable. N and Z are approximately equal. To Ca in N=Z nuclei. 2. Most stable nuclides have even Z and N. 3. Stable nuclides with even numbers are less common. Z and odd. N or even N and odd. Z. 4. P stable nuclides with odd Z and N are rare.
|
number of stable nuclides | ||||
|
odd |
odd | |||
|
odd |
odd | |||
|
odd |
odd |
In kernels with even Z and N nucleons form an ordered structure, which determines their stability. The number of isotopes is smaller in light el. and took him away. in the middle part of the PS, reaching a maximum at Sn (Z=50), which has 10 stable isotopes. Elements with odd. Z stable isotopes no more than 2.
7. Radioactivity and its types Radioactivity - spontaneous transformations of the nuclei of unstable atoms (radionuclides) into stable nuclei of other elements, accompanied by the emission of particles and/or energy radiation. The state of rad does not depend on the chemical. The properties of atoms are determined by the structure of their nuclei. Radioactive decay is accompanied by change. Z and N of the parent atom and leads to the transformation of an atom of one el. into the atom of another el. Also, Rutherford and other scientists have shown that he is glad. the decay is accompanied by the emission of radiation of three different types, a, b, g. a - rays - streams of high-speed particles - He nuclei, b - rays - streams e - , g - rays - electromagnetic waves with high energy and with a shorter λ. Types of radioactivity a-decay- decay by emission of a-particles, it is possible for nuclides with Z> 58 (Ce), and for a group of nuclides with small Z, including 5He, 5Li, 6Be. the a-particle consists of 2 P and 2N, a shift occurs by 2 positions in Z. The original isotope is called parental or maternal, and the newly formed - subsidiaries.
b-decay- has three types: regular b-decay, positronic b-decay and e – capture. Ordinary b-decay- can be considered as the transformation of a neutron into a proton and e - the latter or beta particle - is ejected from the nucleus, accompanied by the emission of energy in the form of g-radiation. The daughter nuclide is an isobar of the parent, but its charge is greater.
There is a series of decays until a stable nuclide is formed. Example: 19 K40 -> 20 Ca40 b - v- Q. Positron b-decay- emission of a positive positron particle b from the nucleus, its formation - the transformation of a nuclear proton into a neutron, positron and neutrino. The daughter nuclide is isobaric but has less charge.
Example, 9 F18 -> 8 O18 b v Q Atoms with an excess of N and located to the right of the nuclear stability zone are b - -radioactive, because in this case, the number N decreases. Atoms to the left of the region of nuclear stability are neutron-deficient, they experience positron decay and their number N increases. Thus, during b- and b-decays there is a tendency for Z and N to change, leading to the daughter nuclides approaching the zone of nuclear stability. e – capture- capture of one of the orbital electrons. There is a high probability of capture from the K-shell, cat. closest to the core. e – capture causes emission of neutrinos from the nucleus. Daughter nuclide yavl. isobaric, and occupies the same position relative to the parent as during positron decay. There is no b - radiation, and when a vacancy in the K shell is filled, X rays are released. At g-radiation neither Z nor A change; when the nucleus returns to its normal state, energy is released in the form g-radiation. Some daughter nuclides of natural isotopes U and Th can decay either by emitting b particles or by a decay. If b-decay occurred first, then a-decay occurred, and vice versa. In other words, these two alternative types decays form closed cycles and always lead to the same final product - stable isotopes of Pb.
8. Geochemical consequences of radioactivity of terrestrial matter. Lord Kelvin (William Thomson) from 1862 to 1899 performed a number of calculations, cat. imposed restrictions on possible age Earth. They were based on consideration of the luminosity of the Sun, the influence of lunar tides and the cooling processes of the Earth. He came to the conclusion that the age of the Earth is 20-40 million years. Rutherford later performed a determination of the age of U min. and obtained values of about 500 million years. Later, Arthur Holmes in his book “The Age of the Earth” (1913) showed the importance of studying radioactivity in geochronology and gave the first GHS. It was based on consideration of data on the thickness of sedimentary sediments and on the content of radiogenic decay products - He and Pb in U-containing minerals. Geochronological scale- scale of natural historical development of the Earth, expressed in numerical units of time. The age of earth's accretion is about 4.55 billion years. A period of up to 4 or 3.8 billion years is the time of differentiation of the planetary interior and the formation of the primary crust; it is called catarchaeum. The longest period of life of Z. and ZK is the Precambrian, cat. extends from 4 billion years to 570 million years, i.e. about 3.5 billion years. The age of the oldest rocks known today exceeds 4 billion years.
9. Geochemical classification of elements V.M. HolschmidtBased on: 1- electrical distribution. between different phases of meteorites - separation during the primary GC differentiation. 2- specific chemical affinity with certain elements (O, S, Fe), 3- structure of electronic shells. The leading elements composing meteorites are O, Fe, Mg, Si, S. Meteorites consist of three main phases: 1) metal, 2) sulfide, 3) silicate. All email are distributed among these three phases in accordance with their relative affinity for O, Fe and S. In Goldschmidt’s classification, the following groups of elements are distinguished: 1) Siderophilous(lovers of iron) – metal. meteorite phase: electrons forming alloys of arbitrary composition with Fe - Fe, Co, Ni, all platinoids (Ru, Rh, Pd, Pt, Re, Os, Ir), and Mo. They often have a native state. These are the transition elements of group VIII and some of their neighbors. Form the inner core of Z. 2) Chalcophilic(copper-loving) - sulfide phase of meteorites: electrons that form natural compounds with S and its analogues Se and Te, also have an affinity for As (arsenic), sometimes they are called (sulfurophilic). They easily turn into a native state. These are elements of secondary subgroups I-II and main subgroups III-VI of groups PS from 4 to 6 period S. The most famous are Cu, Zn, Pb, Hg, Sn, Bi, Au, Ag. Siderophilic el. – Ni, Co, Mo can also be chalcophile with a large amount of S. Fe under reducing conditions has an affinity for S (FeS2). In the modern model of gold, these metals form the outer, sulfur-enriched core of gold.
3) Lithophilic(stone-loving) – silicate phase of meteorites: el., having an affinity for O 2 (oxyphilic). They form oxygen compounds - oxides, hydroxides, salts of oxygen acids - silicates. In compounds with oxygen they have an 8-electron ext. shell. This is the largest group of 54 elements (C, common petrogenic - Si, Al, Mg, Ca, Na, K, elements of the iron family - Ti, V, Cr, Mn, rare - Li, Be, B, Rb, Cs, Sr , Ba, Zr, Nb, Ta, REE, i.e. all others except atmophilic ones). Under oxidizing conditions, iron is oxyphilic - Fe2O3. form the mantle Z. 4) Atmophilic(typical gaseous state) – chondrite matrix: H, N inert gases (He, Ne, Ar, Kr, Xe, Rn). They form the atmosphere of the Earth. There are also such groups: rare earth Y, alkaline, large-ion lithophile elements LILE (K, Rb, Cs, Ba, Sr), high-charge elements or elements with high field strength HFSE (Ti, Zr, Hf, Nb, Ta , Th). Some definitions of email: petrogenic (rock-forming, main) minor, rare, trace elements- from conc. no more than 0.01%. scattered– microel. not forming their own minerals accessory- form accessory min. ore- form ore mines.
10. Basic properties of atoms and ions that determine their behavior in natural systems. Orbital radii - radii of maxima of radial density e – ext. orbitals. They reflect the sizes of atoms or ions in a free state, i.e. outside chem. communications. The main factor is the e – electrical structure, and the more e – shells, the larger the size. For def. the sizes of atoms or ions in an important way. Def. distance from the center of one atom to the center of another, cat. is called the bond length. X-ray methods are used for this. To a first approximation, atoms are considered as spheres, and the “additivity principle” is applied, i.e. It is believed that the interatomic distance is the sum of the radii of the atoms or ions that make up the substance. Then knowing or accepting a certain value as the radius of one el. you can calculate the sizes of all the others. The radius calculated in this way is called effective radius . Coordination number- the number of atoms or ions located in close proximity around the atom or ion in question. The CN is determined by the ratio R k /R a: Valence - the amount of e – donated or attached by an atom during the formation of a chemical. communications. Ionization potential is the energy required to remove e – from an atom. It depends on the structure of the atom and is determined experimentally. The ionization potential corresponds to the voltage of the cathode rays, which is sufficient to ionize an atom of this electron. There may be several ionization potentials, for several e - removed from the external. e – shells. Breaking off each subsequent e requires more energy and is not always possible. Usually they use the ionization potential of the 1st e – , cat. detects periodicity. On the ionization potential curve, alkali metals, which easily lose e – , occupy the minimums on the curve, and inert gases occupy the peaks. With growth atomic number ionization potentials increase in a period and decrease in a group. The reciprocal is the affinity ke – . Electronegativity - the ability to attract e – when entering into connections. The halogens are the most electronegative, the alkali metals the least. Electronegativity depends on the charge of the atomic nucleus, its valence in a given compound and the structure of the e-shells. Attempts have been made repeatedly to express EO in energy units or in conventional units. The EO values change naturally across PS groups and periods. EO is minimal for alkali metals and increases towards halogens. For lithophilic cations, the EO decreases. from Li to Cs and from Mg to Ba, i.e. with increased ionic radius. In chalcophilic el. EO is higher than that of lithophiles from the same PS group. For anions of group O and F, EO decreases down the group and therefore it is maximum for these elements. Email with sharply different meanings EOs form compounds with an ionic type of bond, and with close and high ones - with a covalent bond, with close and low ones - with a metallic type of bond. The Cartledge ionic potential (I) is equal to the ratio of valence to Ri, it reflects the properties of cationogenicity or ionogenicity. V.M. Golshmidt showed that the properties of cationogenicity and anionogenicity depend on the ratio of valence (W) and Ri for ions such as noble gases. In 1928, K. Cartledge called this ratio the ionic potential I. At small values of I el. behaves like a typical metal and cation (alkali and alkaline earth metals), and at large - like a typical non-metal and anion (halogens). It is convenient to depict these relationships graphically. Diagram: ionic radius - valence. The magnitude of the ionic potential allows one to judge the mobility of the electron. in an aquatic environment. Email with low and high values of I they are the most mobile easily (with low values they pass into ionic solutions and migrate, with high values they form complex soluble ions and migrate), and with intermediate values they are inert. Main types of chemicals bonds, character of bonds in the main groups of minerals. Ionic– an image due to the attraction of ions with opposite charges. (with a large difference in electronegativity) Ionic bonding predominates in most min. ZK - oxides and silicates, this is the most common type of bond also in hydro- and atmospheres. The connection ensures easy dissociation of ions in melts, solutions, gases, due to which wide migration of chemicals occurs. El., their dispersion and concentration in the earth's geospheres. Covalent – noun due to the interaction of e – used by different atoms. Typical for email. with an equal degree of attraction e –, i.e. EO. Characteristic for liquid and gaseous substances (H2O, H2, O2, N2) and less for crystals. Covalent bonds characterize sulfides, related compounds As, Sb, Te, as well as monoel. non-metal compounds - graphite, diamond. Covalent compounds are characterized by low solubility. Metal- a special case of a covalent bond, when each atom shares its e - with all neighboring atoms. e – capable of free movement. Typical for native metals (Cu, Fe, Ag, Au, Pt). Many min. have a connection, cat. refers partly to ionic, partly to covalent. In sulfide min. The covalent bond is maximally manifested; it occurs between the metal atoms and S, and the metal bond occurs between the metal atoms (metal luster of sulfides). Polarization - This is the effect of distortion of the e-cloud of an anion by a small cation with a high valence so that the small cation, attracting a large anion to itself, reduces its effective R, itself entering its e-cloud. Thus, the cation and anion are not regular spheres, and the cation causes deformation of the anion. The higher the charge of the cation and the smaller its size, the stronger effect polarization. And the larger the size of the anion and its negative charge, the more it is polarized - deformed. Lithophilic cations (with 8 electron shells) cause less polarization than ions with complementary shells (such as Fe). Chalcophile ions with large ordinal numbers and high valence call the strongest polarization. This is associated with the formation of complex compounds: 2-, , 2-, 2-, cat. soluble and yavl. the main transporters of metals in hydrothermal solutions.
11.State (form of location) email. in nature. In GC the following are distinguished: min. (crystalline phases), impurities in min., various forms of dispersed state; email location form in nature carries information about the degree of ionization, chemical characteristics. email connections in phases, etc. In-vo (el.) is in three main forms. The first is the end atoms, the image. stars are different. types, gas nebulae, planets, comets, meteorites and cosmos. TV particles in. Concentration degree The substance is different in all bodies. The most diffuse states of atoms in gaseous nebulae are held by gravitational forces or are on the verge of overcoming them. The second is scattered atoms and molecules, an image of interstellar and intergalactic gas, consisting of free atoms, ions, molecules, e – . The amount of it in our Galaxy is significantly less than that which is concentrated in stars and gaseous nebulae. Interstellar gas is located at different levels. stages of rarefaction. Third - intensively migrating, flying at enormous speed atomic nuclei and the elementary particles that make up cosmic rays. IN AND. Vernadsky identified the main four forms of occurrence of chemicals. Email in the Earth's Earth and on its surface: 1. rocks and minerals (solid crystalline phases), 2. magma, 3. dispersed state, 4. living matter. Each of these forms is distinguished by a special state of their atoms. Noun and other selection of forms of location of email. in nature, depending on specific holy elements themselves. A.I. Perelman highlighted mobile and inert forms finding chemical Email in the lithosphere. By his definition, movable form represents such a state of chemistry. Email in gp, soils and ores, being in the cat. Email can easily move into the solution and migrate. Inert form represents such a state in mineral deposits, ores, weathering crust and soils, in cat. Email in this situation, it has a low migration ability and cannot move into the region and migrate.
12.Internal factors of migration.
Migration- movement of chemicals Email in geospheres Z, leading to their dispersion or conc. Clarke - medium conc. in the main types of gp ZK of each chemical. Email can be considered as the state of its equilibrium under the conditions of a given chemical. environment, deviation from cat. is gradually reduced by the migration of this electricity. Under terrestrial conditions, the migration of chemicals. Email occurs in any medium - TV. and gaseous (diffusion), but easier in a liquid medium (in melts and aqueous solutions). At the same time, the forms of migration of chemicals. Email are also different - they can migrate in atomic (gases, melts), ionic (solutions, melts), molecular (gases, solutions, melts), colloidal (solutions) forms and, in the form of clastic particles (air and water environment ). A.I. Perelman distinguishes four types of chemical migration. El.: 1.mechanical, 2.physical-chemical, 3.biogenic, 4.technogenic. The most important internal factors: 1. Thermal properties of electricity, i.e. their volatility or refractoriness. El., having a condensation temperature of more than 1400 o K are called refractory platinoids, lithophilic - Ca, Al, Ti, Ree, Zr, Ba, Sr, U, Th), from 1400 to 670 o K - moderately volatile. [lithophilic – Mg, Si (moderately refractory), many chalcophilic, siderophilic – Fe, Ni, Co ],< 670 o K – летучими (атмофильные). На основании этих св-в произошло разделение эл. по геосферам З. При магм. процессе в условиях высоких Т способность к миграции будет зависеть от возможности образования тугооплавких соединений и, нахождения в твердой фазе. 2. Хим. Св-ва эл. и их соединений. Атомы и ионы, обладающие слишком большими или слишком малыми R или q, обладают и повышенной способностью к миграции и перераспределению. Хим. Св-ва эл. и их соединений приобретают все большее значение по мере снижения T при миграции в водной среде. Для литофильных эл. с низким ионным потенциалом (Na, Ca, Mg) в р-рах хар-ны ионные соединения, обладающие высокой раствор-ю и высокими миграционными способностями. Эл. с высокими ионными потенциалами образуют растворимые комплексные анионы (С, S, N, B). При низких Т высокие миграционные способности газов обеспечиваются слабыми молекулярными связями их молекул. Рад. Св-ва, опред-ие изменение изотопного состава и появление ядер других эл.
, meteoroid, asteroid, their fragments, or other meteoroids.
A celestial body flying through the Earth's atmosphere and leaving a bright luminous trail in it, regardless of whether it flies through the upper layers of the atmosphere and goes back into outer space, burns up in the atmosphere, or falls to Earth, can be called either a meteor or a bolide . Meteors are considered bodies no brighter than 4th magnitude, and fireballs - brighter than 4th magnitude, or bodies whose angular dimensions are distinguishable.
A solid body of cosmic origin that fell to the surface of the Earth is called a meteorite.
A crater (astrobleme) may form at the site where a large meteorite falls. One of the most famous craters in the world is Arizona. It is assumed that the largest meteorite crater on Earth is Wilkes Earth Crater (diameter about 500 km).
Other names for meteorites: aerolites, siderolites, uranolites, meteorolites, baituloi, sky, air, atmospheric or meteor stones, etc.
Phenomena similar to the fall of a meteorite on other planets and celestial bodies are usually called simply collisions between celestial bodies.
The process of meteorites falling to Earth
The meteor body enters the Earth's atmosphere at a speed of about 11-25 km/sec. At this speed, it begins to warm up and glow. Due to ablation (burning and blowing away by the oncoming flow of particles of the meteoroid body), the mass of the body that reaches the ground may be less, and in some cases significantly less than its mass at the entrance to the atmosphere. For example, a body that enters the Earth's atmosphere at a speed of 25 km/s or more burns up almost completely. At such a speed of entry into the atmosphere, out of tens and hundreds of tons of initial mass, only a few kilograms or even grams of matter reach the ground. Traces of the combustion of a meteoroid in the atmosphere can be found along almost the entire trajectory of its fall.
If the meteor body does not burn up in the atmosphere, then as it slows down it loses the horizontal component of its speed. This results in a change in the trajectory of the fall from often almost horizontal at the beginning to almost vertical at the end. As it slows down, the glow of the meteorite decreases and it cools down (they often indicate that the meteorite was warm and not hot when it fell).
In addition, the meteor body may break into fragments, resulting in a Meteor Shower.
Classification of meteorites
Classification by composition
- stone
- chondrites
- carbonaceous chondrites
- ordinary chondrites
- enstatite chondrites
- chondrites
- iron-stone
- palasites
- mesosiderites
- iron
The most common meteorites are stony meteorites (92.8% of falls). They consist mainly of silicates: olivines (Fe, Mg)2SiO4 (from fayalite Fe2SiO4 to forsterite Mg2SiO4) and pyroxenes (Fe, Mg)SiO3 (from ferrosilite FeSiO3 to enstatite MgSiO3).
The vast majority of stony meteorites (92.3% of stony meteorites, 85.7% of total falls) are chondrites. They are called chondrites because they contain chondrules - spherical or elliptical formations of predominantly silicate composition. Most chondrules are no more than 1 mm in diameter, but some can reach several millimeters. Chondrules are found in a detrital or finely crystalline matrix, and often the matrix differs from chondrules not so much in composition as in crystalline structure. The composition of chondrites almost completely replicates the chemical composition of the Sun, with the exception of light gases such as hydrogen and helium. Therefore, it is believed that chondrites formed directly from the protoplanetary cloud that surrounded and surrounded the Sun, through the condensation of matter and the accretion of dust with intermediate heating.
Achondrites make up 7.3% of stony meteorites. These are fragments of protoplanetary (and planetary?) bodies that have undergone melting and differentiation by composition (into metals and silicates).
Iron meteorites are composed of an iron-nickel alloy. They account for 5.7% of falls.
Iron silicate meteorites have a composition intermediate between stony and iron meteorites. They are relatively rare (1.5% incidence).
Achondrites, iron and iron-silicate meteorites are classified as differentiated meteorites. They presumably consist of matter that has undergone differentiation as part of asteroids or other planetary bodies. It was previously believed that all differentiated meteorites were formed by the rupture of one or more large bodies, such as the planet Phaeton. However, an analysis of the composition of different meteorites showed that they were more likely formed from the debris of many large asteroids.
Classification by detection method
- falls (when a meteorite is found after observing its fall in the atmosphere);
- finds (when the meteorite origin of the material is determined only by analysis);
Traces of extraterrestrial organics in meteorites
Coal complex
Carbonaceous (carbonaceous) meteorites have one important feature - the presence of a thin glassy crust, apparently formed under the influence of high temperatures. This crust is a good heat insulator, thanks to which minerals that cannot withstand strong heat, such as gypsum, are preserved inside carbonaceous meteorites. Thus, it became possible, when studying the chemical nature of such meteorites, to detect in their composition substances that, under modern earthly conditions, are organic compounds of a biogenic nature ( Source: Rutten M. Origin of life (naturally). - M., Publishing House "Mir", 1973) :
- Saturated hydrocarbons
- Isoprenoids
- n-Alkanes
- Cycloalkanes
- Aromatic hydrocarbons
- Naphthalene
- Alkybenzenes
- Acenaphthenes
- Pyrene
- Carboxylic acids
- Fatty acid
- Benzenecarboxylic acids
- Hydroxybenzoic acids
- Nitrogen compounds
- Pyrimidines
- Purines
- Guanylurea
- Triazines
- Porphyrins
The presence of such substances does not allow us to unambiguously declare the existence of life outside the Earth, since theoretically, if certain conditions were met, they could be synthesized abiogenically.
On the other hand, if the substances found in meteorites are not products of life, then they may be products of pre-life - similar to that which once existed on Earth.
"Organized Elements"
When studying stony meteorites, so-called “organized elements” are discovered - microscopic (5-50 microns) “single-cell” formations, often having clearly defined double walls, pores, spines, etc. ( Source: Same)
It is not an indisputable fact that these fossils are the remains of some form of extraterrestrial life. But, on the other hand, these formations have such a high degree of organization that is usually associated with life ( Source: Same).
In addition, such forms have not been found on Earth.
A feature of “organized elements” is also their large number: per 1g. The substances of the carbonaceous meteorite account for approximately 1800 “organized elements”.
Large modern meteorites in Russia
- Tunguska phenomenon (at the moment, the exact meteorite origin of the Tunguska phenomenon is unclear. For details, see the article Tunguska meteorite). Fell on June 30 this year in the Podkamennaya Tunguska river basin in Siberia. The total energy is estimated at 15−40 megatons of TNT equivalent.
- Tsarevsky meteorite (meteor shower). Fell on December 6 near the village of Tsarev, Volgograd region. This is a rock meteorite. The total mass of the collected fragments is 1.6 tons over an area of about 15 square meters. km. The weight of the largest fallen fragment was 284 kg.
- Sikhote-Alin meteorite (total mass of fragments is 30 tons, energy is estimated at 20 kilotons). It was an iron meteorite. Fell in the Ussuri taiga on February 12.
- Vitimsky car. Fell in the area of the villages of Mama and Vitimsky, Mamsko-Chuysky district, Irkutsk region, on the night of September 24-25. The event had a great public resonance, although the total energy of the meteorite explosion is apparently relatively small (200 tons of TNT equivalent, with an initial energy of 2.3 kilotons), the maximum initial mass (before combustion in the atmosphere) is 160 tons, and the final mass of the fragments is about several hundred kilograms.
The discovery of a meteorite is a rather rare occurrence. The Meteoritics Laboratory reports: “In total, only 125 meteorites have been found on the territory of the Russian Federation over 250 years.”
The only documented case of a meteorite hitting a person occurred on November 30 in Alabama. The meteorite, weighing about 4 kg, crashed through the roof of the house and ricocheted Anna Elizabeth Hodges on the arm and thigh. The woman received bruises.
Other Interesting Facts about meteorites:
Individual meteorites
- Channing
- Chainpur
- Beeler
- Arcadia
- Arapahoe
Notes
Links
Meteorite crash sites Google Maps KMZ(KMZ tag file for Google Earth)
- Museum of Extraterrestrial Matter RAS (meteorite collection)
- Peruvian chondrite (commentary by astronomer Nikolai Chugay)
see also
- Meteor craters or astroblemes.
- Portal:Meteorites
- Moldavite
Wikimedia Foundation. 2010.
Meteorites are small iron, stone or iron-stone space objects that regularly fall to the surface of planets solar system, including the Earth. Outwardly, they are not much different from stones or pieces of iron, but they conceal many mysteries from the history of the universe. Meteorites help scientists uncover the secrets of the evolution of celestial bodies and study processes occurring far beyond our planet.
By analyzing their chemical and mineral composition, it is possible to trace patterns and connections between meteorites various types. But each of them is unique, with qualities inherent only to this body of cosmic origin.
Types of meteorites by composition:
1. Stone:
Chondrites;
Achondrites.
2. Iron-stone:
Pallasites;
Mesosiderites.
3. Iron.
Octahedrites
Ataxites
4. Planetary
Martian
Origin of meteorites
Their structure is extremely complex and depends on many factors. Studying all known varieties of meteorites, scientists came to the conclusion that they are all closely related at the genetic level. Even taking into account significant differences in structure, mineral and chemical composition, they are united by one thing - origin. All of them are fragments of celestial bodies (asteroids and planets), moving in outer space at high speed.
Morphology
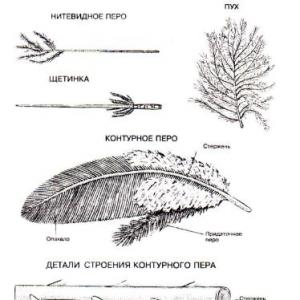
To reach the surface of the Earth, a meteorite needs to travel a long way through the layers of the atmosphere. As a result of significant aerodynamic load and ablation (high-temperature atmospheric erosion), they acquire characteristic external features:
Oriented conical shape;
Melting crust;
Special surface relief.
A distinctive feature of real meteorites is the melting crust. It can differ quite significantly in color and structure (depending on the type of body of cosmic origin). In chondrites it is black and matte, in achondrites it is shiny. In rare cases, the fusion bark may be light and translucent.
With a long stay on the surface of the Earth, the surface of the meteorite is destroyed under the influence of atmospheric influences and oxidation processes. For this reason, a significant part of bodies of cosmic origin after a certain time is practically no different from pieces of iron or stones.
Another distinctive external sign, which a real meteorite has, is the presence on the surface of depressions called piezoglypts or regmaglypts. Resembles fingerprints on soft clay. Their size and structure depend on the conditions of movement of the meteorite in the atmosphere.
Specific gravity
1. Iron - 7.72. The value can vary in the range of 7.29-7.88.
2. Pallasites – 4.74.
3. Mesosiderites – 5.06.
4. Stone - 3.54. The value can vary in the range of 3.1-3.84.
Magnetic and optical properties
Due to the presence of a significant amount of nickel iron, a real meteorite exhibits its unique magnetic properties. This is used to verify the authenticity of a body of cosmic origin and allows indirect judgment of the mineral composition.
The optical properties of meteorites (color and reflectivity) are less pronounced. They appear only on the surfaces of fresh fractures, but over time due to oxidation they become less noticeable. Comparing the average values of the brightness coefficient of meteorites with the albedo of celestial bodies of the solar system, scientists came to the conclusion that some planets (Jupiter, Mars), their satellites, as well as asteroids are similar in their optical properties to meteorites.
Chemical composition of meteorites
Considering the asteroidal origin of meteorites, their chemical composition can differ quite significantly between objects of different types. This has a significant impact on the magnetic and optical properties, as well as the specific gravity of bodies of cosmic origin. The most common chemical elements in meteorites are:
1. Iron (Fe). Is the main one chemical element. Occurs in the form of nickel iron. Even stony meteorites have an average Fe content of 15.5%.
2. Nickel (Ni). It is part of nickel iron, as well as minerals (carbides, phosphides, sulfides and chlorides). Compared to Fe, it is 10 times less common.
3. Cobalt (Co). Not found in pure form. Compared to nickel, it is 10 times less common.
4. Sulfur (S). Part of the mineral troilite.
5. Silicon (Si). It is part of the silicates that form the bulk of stone meteorites.
3. Orthorhombic pyroxene. Often found in stony meteorites, it is the second most common among silicates.
4. Monoclinic pyroxene. It is found rarely and in small quantities in meteorites, with the exception of achondrites.
5. Plagioclase. A common rock-forming mineral belonging to the feldspar group. Its content in meteorites varies widely.
6. Glass. It is the main component of stone meteorites. Contained in chondrules and also found as inclusions in minerals.